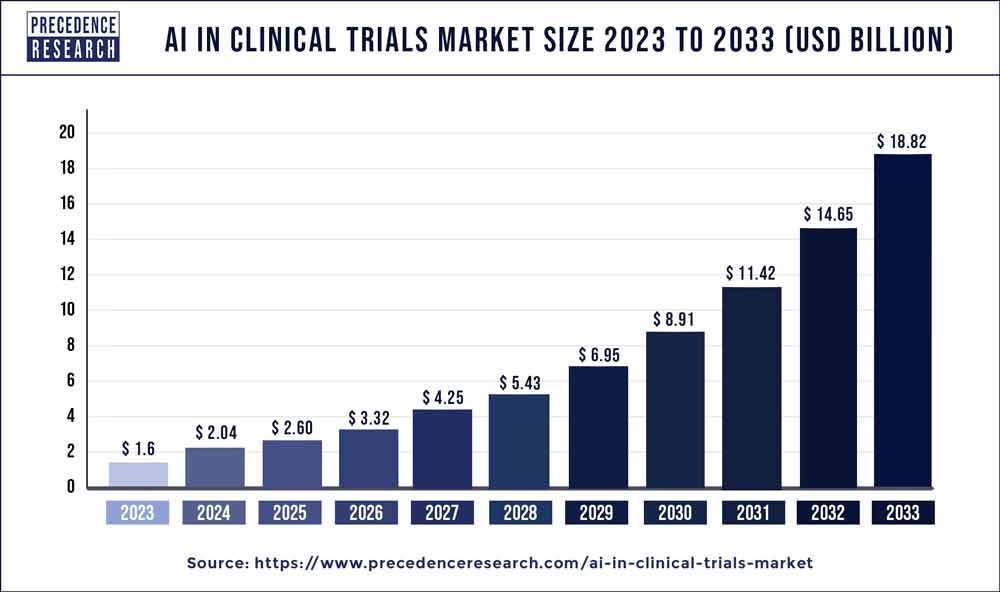
The global AI in clinical trials market size is projected to hit around USD 18.82 billion by 2033, poised to grow at a CAGR of 28% from 2024 to 2033.
Key Ponits
- North America held the dominating share of the AI in clinical trials market in 2023.
- By offering, the services segment held the largest market share in 2023.
- By technology, the deep learning segment held the dominating market share in 2023.
- By application, the infectious disease segment dominated the market in 2023.
- By end-user, the pharmaceutical segment held the largest share of the market in 2023; the segment is observed to sustain dominance throughout the forecast period.
Introduction:
The integration of Artificial Intelligence (AI) in clinical trials represents a paradigm shift in the healthcare industry, revolutionizing the way we conduct and perceive medical research. As traditional methods encounter challenges and limitations, AI emerges as a transformative force, offering unprecedented opportunities to enhance efficiency, accuracy, and overall effectiveness in clinical trials. This dynamic intersection of technology and healthcare holds the promise of accelerating drug discovery, optimizing patient recruitment, and streamlining data analysis, ultimately paving the way for more agile and cost-effective clinical research.
Get a Sample: https://www.precedenceresearch.com/sample/3743
Growth Factors:
Several key factors contribute to the remarkable growth of AI in clinical trials. Firstly, AI facilitates the analysis of vast datasets with incredible speed and precision, uncovering patterns and correlations that might elude conventional methods. This data-driven approach enables researchers to make informed decisions, identify potential risks, and streamline the drug development process. Additionally, AI-powered predictive analytics enhance patient stratification, ensuring more targeted and personalized treatment plans, ultimately improving the success rates of clinical trials.
Moreover, the increasing complexity of clinical trials necessitates sophisticated tools for data management and interpretation. AI solutions excel in handling intricate datasets, offering a level of efficiency and accuracy crucial for navigating the intricacies of modern clinical research. The ability to process real-time data also contributes to adaptive trial designs, allowing for swift adjustments based on emerging insights, ultimately reducing trial duration and costs.
AI in Clinical Trials Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 28% |
| Global Market Size in 2023 | USD 1.6 Billion |
| Global Market Size by 2033 | USD 18.82 Billion |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Offering, By Technology, By Application, and By End-user |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Recent Developments
- In September 2023, a global pioneer in providing individuals and institutions with reliable intelligence to change the world, Clarivate Plc announced the creation of an Academia & Government Innovation Incubator. This will quicken its approach to fostering creativity, using AI, and launching cutting-edge products for its academic clients and users.
- In July 2023, by advancing the first medication identified and created by generative AI into Phase II clinical trials involving humans, Insilico Medicine has set a new standard in artificial intelligence drug research. The primary program, INS018_055, is a pan-fibrotic inhibitor that may be the first of its kind. Insilico’s moonshot medication unequivocally proves the viability of the company’s end-to-end AI drug development platform, Pharma. AI.
AI in Clinical Trials Market Dynamics
Drivers:
The drivers propelling the adoption of AI in clinical trials are diverse and impactful. One primary driver is the demand for accelerated drug development. AI expedites various stages of clinical trials, from target identification to patient recruitment and monitoring, resulting in a substantial reduction in the time required to bring new therapies to market. The efficiency gains translate into cost savings for pharmaceutical companies, incentivizing the industry to invest in and embrace AI technologies.
Another significant driver is the growing emphasis on patient-centric approaches. AI enables a more nuanced understanding of patient characteristics and responses to treatments, fostering the development of personalized medicine. Tailored interventions not only enhance patient outcomes but also contribute to the overall success of clinical trials by minimizing adverse events and improving participant retention rates.
Restraints:
Despite the promising trajectory, the integration of AI in clinical trials is not without challenges. A notable restraint is the inherent complexity of AI algorithms and their interpretability. Regulatory bodies and stakeholders often grapple with understanding the decision-making processes of AI systems, raising concerns about transparency and accountability. Overcoming this hurdle requires concerted efforts in developing standardized guidelines and ensuring a harmonized regulatory framework that addresses the unique challenges posed by AI in the context of clinical trials.
Additionally, the initial investment required for implementing AI infrastructure and training personnel presents a financial barrier for some organizations. The transition from traditional methodologies to AI-driven processes demands a commitment to restructuring existing workflows and providing comprehensive training, which can be resource-intensive.
Opportunities:
Amidst the challenges, numerous opportunities arise that can propel the integration of AI in clinical trials to new heights. One such opportunity lies in the collaborative efforts between industry stakeholders, regulatory bodies, and technology developers. Establishing clear guidelines and standards for the application of AI in clinical trials fosters a conducive environment for innovation while ensuring the safety and ethical conduct of research.
Furthermore, the ongoing advancements in AI technologies, such as machine learning and natural language processing, open avenues for novel applications in clinical trial operations. Predictive modeling, risk assessment, and identification of potential safety issues are areas where AI can significantly contribute, providing valuable insights that streamline decision-making processes and enhance trial outcomes.
Read Also: Network Engineering Services Market Size to Rise $97 Bn by 2033
AI in Clinical Trials Market Companies
- AiCure
- Antidote Technologies
- Deep 6 AI
- Mendel.ai
- Phesi
- Saama Technologies
- Signant Health
- Trials.ai
- Innoplexus
- IQVIA
- Median Technologies
- Medidata
Segments Covered in the Report
By Offering
- Software
- Services
By Technology
- Machine learning
- Deep learning
- Supervised
By Application
- Cardiovascular
- Metabolic
- Oncology
- Infectious diseases
By End-user
- Pharma
- Biotech
- CROs
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/
