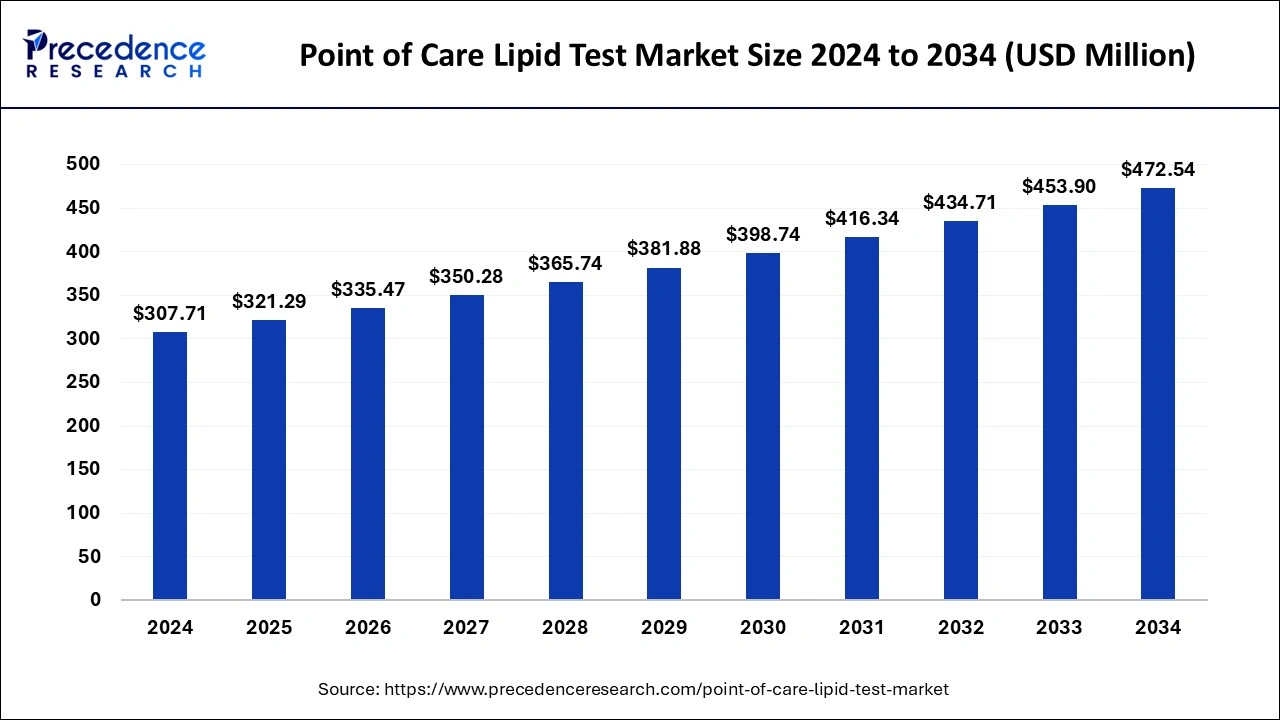
The global point of care lipid test market size accounted for USD 294.70 million in 2023 and is predicted to grow around USD 453.90 million by 2033, growing at a CAGR of 4.41% from 2024 to 2033.
Key Points
- North America held a significant share of in the point of care lipid test market in 2023 with 38.6%.
- Asia Pacific is anticipated to experience the highest growth in the upcoming years.
- By product, the consumables type segment has accounted market share of 57% in 2023.
- By application, in 2023, the endogenous hyperlipidemia segment dominated the market with 46% revenue share.
- By end use, the diagnostic centers segment has held largest market share of 56% in 2023.
The point of care (POC) lipid test market is an area of the medical diagnostics field that focuses on providing rapid lipid profiling tests at or near the site of patient care. This includes tests for cholesterol and triglyceride levels that can be used for screening, monitoring, and managing cardiovascular risks and diseases. POC lipid tests offer convenience and faster results compared to traditional laboratory tests, which is a significant advantage for healthcare providers and patients.
Get a Sample: https://www.precedenceresearch.com/sample/4076
Overview
The POC lipid test market has grown steadily in recent years due to increasing awareness about cardiovascular diseases (CVDs) and their associated risk factors. These tests allow healthcare professionals to quickly assess lipid profiles and take immediate action based on the results, which can lead to better patient outcomes and more efficient healthcare management. POC lipid tests are especially useful in primary care settings, emergency rooms, and remote locations where laboratory access may be limited.
Growth Factors
Several factors contribute to the growth of the POC lipid test market:
- Rising Prevalence of Cardiovascular Diseases: The increasing incidence of CVDs globally drives the demand for lipid tests for early diagnosis and management of risk factors.
- Technological Advancements: Innovations in testing technologies have made POC lipid tests more accurate, reliable, and user-friendly. This, in turn, increases their adoption in various healthcare settings.
- Increasing Awareness: Greater awareness of the importance of regular lipid profiling for cardiovascular health encourages individuals and healthcare providers to use POC lipid tests more frequently.
- Aging Population: An aging population worldwide increases the prevalence of CVDs, necessitating regular lipid monitoring, which in turn boosts the market for POC lipid tests.
- Rise of Chronic Diseases: With the increasing prevalence of chronic diseases such as diabetes and obesity, POC lipid tests become an essential tool for monitoring and managing associated risks.
Region Insights
The market is segmented geographically into North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa.
- North America: North America is one of the leading markets for POC lipid tests due to high healthcare expenditure, advanced healthcare infrastructure, and high prevalence of CVDs. Additionally, the region has a strong focus on preventive healthcare and early diagnostics.
- Europe: Europe follows closely behind North America in market share, benefiting from advanced medical infrastructure, widespread awareness, and high adoption rates of new technologies.
- Asia Pacific: The Asia Pacific region is expected to witness significant growth due to increasing healthcare expenditure, growing awareness, and a rising number of individuals with CVDs and other chronic diseases. Countries like China and India present immense opportunities for market expansion.
- Latin America and the Middle East & Africa: These regions are also experiencing growth, though at a slower rate compared to North America and Europe. As healthcare infrastructure improves and awareness grows, these markets could present opportunities for further expansion.
Point of Care Lipid Test Market Scope
| Report Coverage | Details |
| Global Market Size in 2023 | USD 294.70 Million |
| Global Market Size in 2024 | USD 307.71 Million |
| Global Market Size by 2033 | USD 453.90 Million |
| Growth Rate from 2024 to 2033 | CAGR of 4.41% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Product Type, By Application, and By End-user |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Point of Care Lipid Test Market Dynamics
Drivers
Key drivers in the POC lipid test market include:
- Increased Demand for Preventive Healthcare: The shift towards preventive healthcare and early detection of cardiovascular risk factors drives demand for POC lipid tests.
- Convenience and Speed: The ability to obtain rapid results at the point of care allows for immediate decision-making and action, which is especially crucial in acute care settings.
- Patient Empowerment: Patients can take a more active role in managing their health by using POC lipid tests at home or in primary care clinics, leading to better engagement in their health management.
- Cost-Effectiveness: POC lipid tests can reduce the need for multiple doctor visits and hospitalizations, resulting in cost savings for both patients and healthcare systems.
Opportunities
Opportunities in the POC lipid test market include:
- Product Innovation: Developing more accurate, reliable, and user-friendly POC lipid tests will continue to drive market growth.
- Expansion in Emerging Markets: The Asia Pacific, Latin America, and Middle East & Africa regions offer vast opportunities for market expansion due to rising healthcare infrastructure and growing awareness of CVDs.
- Integration with Digital Health: The integration of POC lipid tests with digital health platforms can improve data management and patient engagement, offering new avenues for market growth.
- Collaborations and Partnerships: Strategic collaborations between key players, research institutions, and healthcare providers can lead to the development of more advanced testing technologies and wider market penetration.
Challenges
Despite the growth potential, the POC lipid test market faces several challenges:
- Regulatory Hurdles: Meeting regulatory requirements and obtaining approvals can be time-consuming and expensive, impacting the speed of product launches.
- Competition from Traditional Labs: Traditional laboratory-based lipid tests still dominate the market in some regions, posing a challenge to the growth of POC lipid tests.
- Cost of Advanced Testing Technologies: Developing advanced POC lipid tests can be costly, and higher prices may limit their adoption, particularly in low-income regions.
- Data Management Concerns: The integration of POC lipid tests with digital health records raises concerns about data security and patient privacy.
Read Also: Electric Vehicle Thermal Management Market Size, Trends Report By 2033
Recent Developments
- In October 2022 Genes2Me Pvt. Ltd launched Rapi-Q- Point of Care RT PCR solution for human papillomavirus (HPV) and tuberculosis. The device is easy to use and gives faster results in less than 45 minutes. This CE-IVD-marked POC solution delivers superior performance, high sensitivity, and stable detection.
- In March 2022, Visby Medical announced that it received funding of USD 25.5 million from the U.S. Biomedical Advanced Research and Development Authority to develop a rapid flu-COVID-19 PCR test for home use. At present, the test is in the under-developing phase, and the design is ready as a PCR device that can detect COVID-19, influenza A, and B from a single sample.
- In March 2022, Canada-based Company BioLytical Laboratories Inc. received a CE marking for the iStatis COVID-19 Antigen Home Test.
- In May 2022, Qiagen Inc. launched the NeuMoDxHSV 1/2 Quant Assay for the quantification and differentiation of herpes simplex virus type 1 (HSV-1) DNA and herpes simplex virus type 2 (HSV-2) with approval from the European Commission. The emergence of this assay is allowing the company to expand its product portfolio in laboratory testing, which ultimately helps the market grow owing to the innovative tests.
- In March 2022, Mindray launched the BC-700 Series, a hematology analyzer series that assists in both blood count and erythrocyte sedimentation rate tests.
Point of Care Lipid Test Market Com[panies
- Callegari Sinocare Inc.
- Abbott Laboratories
- Mico Bio Med
- Nova Biomedical Corporation
- VivaChek Biotech (Hangzhou) Co., Ltd.
- F. Hoffmann-La Roche Ltd.
- Zoetis Inc.
- Menarini Group
- SD Biosensor, Inc.
Segments Covered in the Report
By Product Type
- Devices
- Consumables
By Application
- Endogenous Hyperlipemia
- Combined Hyperlipidemia
- Familial Hypercholesterolemia
- Others
By End-user
- Hospitals And Clinics
- Diagnostic Laboratories
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/
