Nitinol-Based Medical Device Market Key Takeaways
- North America dominated the nitinol-based medical device market in 2024.
- Asia Pacific is expected to grow at a CAGR of 8.06% between 2025 and 2034.
- By product type, the retriever device segment dominated the market in 2024.
- By product type, the catheters segment is expected to grow at the highest CAGR during the projection period.
- By application, the cardiovascular segment led the market in 2024.
- By end-use, the hospitals segment held the dominant market share in 2024.
- By end-use, the ambulatory surgical centers segment is projected to grow at a CAGR of 8.1% between 2025 and 2034.
Nitinol-Based Medical Device Market Overview
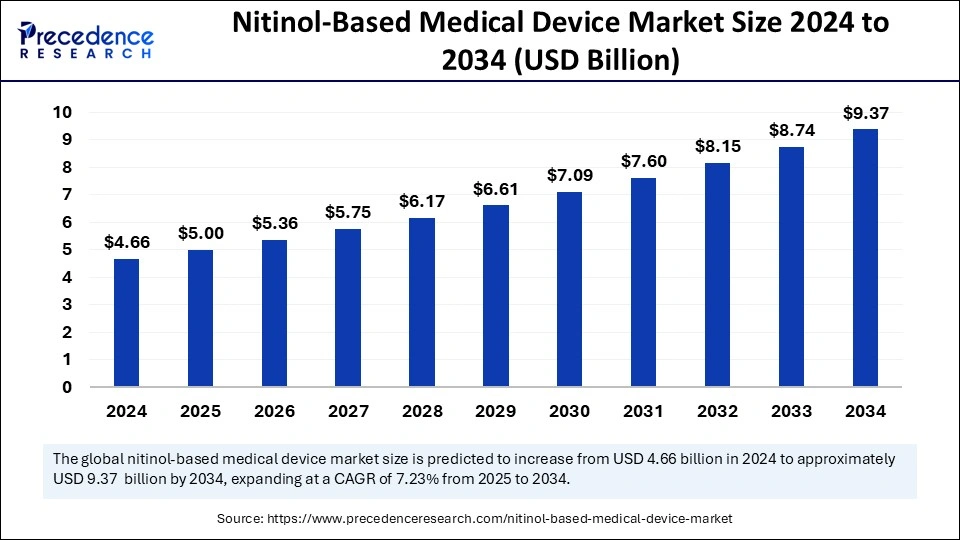
The Nitinol-based medical device market is experiencing robust growth, fueled by innovations in minimally invasive surgical devices. The unique characteristics of Nitinol, including its ability to return to a predetermined shape and its superelasticity, make it an ideal material for devices used in procedures that require precision and flexibility.
The nitinol-based medical device market is witnessing rapid growth due to the rising advancements in nitinol processing and manufacturing technologies. Advancements in manufacturing technologies led to the development of more sophisticated nitinol-based medical devices. Being a metallic biomaterial and having unique properties such as superelasticity, shape memory, and greater damping characteristics make nitinol a preferred material for use in medical devices. It is a very useful material in orthopedic implants and other surgical instruments.
Despite the greater initial nickel dissolution, it does not induce toxic effects, reduce cell proliferation, or prevent the growth of cells in contact with the metal surface. Nitinol is potentially used in cardiac devices like vena cava filters, stents, guidewires, and heart valves. The applications of nitinol in other equipment areas are rising, predominantly for products intended for usage in minimally invasive procedures. Nitinol is a well-known and common engineering material in the healthcare sector.
Drivers
Key drivers of this market segment include the increasing preference for minimally invasive surgeries, which offer benefits such as reduced recovery times, lower risk of complications, and shorter hospital stays. The demand for advanced medical devices that can navigate complex anatomical structures has led to the adoption of Nitinol-based products in various specialties, including cardiology, neurology, and gastroenterology.
Opportunities
The integration of Nitinol-based devices with digital health technologies presents significant opportunities for market expansion. The development of smart implants and devices that can provide real-time data and feedback is an emerging trend. Additionally, the growing focus on personalized medicine and patient-specific devices is expected to drive innovation in the design and application of Nitinol-based medical devices.
Challenges
Challenges in this market include the need for specialized manufacturing processes and equipment to handle Nitinol’s unique properties. The high cost of raw materials and the complexity of device design can lead to increased production costs. Moreover, ensuring consistent quality and performance of Nitinol-based devices requires stringent quality control measures, which can be resource-intensive.
Regional Insights
North America remains the largest market for Nitinol-based medical devices, supported by a strong healthcare system and a high rate of adoption of advanced medical technologies. Europe is also a significant market, with ongoing research and development activities focused on expanding the applications of Nitinol. The Asia-Pacific region is emerging as a key growth area, with countries like China and India investing in healthcare infrastructure and increasing access to advanced medical procedures.
Recent Developments
Recent advancements in the market include the approval of new Nitinol-based devices by regulatory authorities, facilitating their entry into clinical practice. For example, in February 2022, Cordis Health received FDA approval for a new delivery system for its S.M.A.R.T. Control Nitinol Stent, allowing for trans-radial access. Additionally, companies are investing in research to develop Nitinol devices with enhanced properties, such as improved fatigue resistance and biocompatibility, to expand their use in various medical applications.
Nitinol-Based Medical Device Market Companies
- Medtronic plc
- Terumo Corporation
- Cordis
- Zimmer Biomet Holding, Inc.
- Becton, Dickinson, and Company
- W.L. Gore & Associates Inc
- Boston Scientific Corporation
- Arthrex Inc
- Cook Group
- Solventum
Segments Covered in the Report
By Product Type
- Stents
- Guidewires
- Retriever Device
- Catheters
- Others
By Application
- Dentistry
- Urology
- Others
- Cardiovascular
By End-use
- Hospitals
- Ambulatory Surgical Centers
- Others
By Region
- North America
- Europe
- Asia Pacific
- Middle East & Africa
- Latin America
Ready for more? Dive into the full experience on our website!
